Cross-contamination in pharmaceutical manufacturing has become a huge concern not only to experts involved in the therapeutic market and pharma industry as a whole, but to the average person as well. It becomes a major topic of discussion among various communities. But what exactly is this? In essence, that is the ‘infection’, so to speak, of any material in process (starting material or intermediate product) or a finished drug with another material in process or pharmaceutical. It is critical for manufacturers to have appropriate procedures in place in order to prevent risks of contamination and to prove that no contamination has occurred by delivering detailed documentation. The dangers and negative effects this process may cause are many. Taking measures against cross-contamination, then, becomes something of extreme importance. It is crucial as such preventive actions can retain the quality of products. But what is even more significant, they can minimize any life-threatening reactions which may result from the potential consumption of cross-contaminated medicaments.
Of course, there are certain challenges when it comes to adopting actions designed to avoid mix-ups. And one of these difficulties derives from the fact that many production lines for different active medical materials and substances are run simultaneously in production plants. It is a common practice, frequently conducted because of its cost-efficiency and time-efficacy. However, the question here is, is it completely safe? No, it isn’t. Such saving on resources and time quite often turns into a compromise on safety. And it does more harm than good. In other words, running production lines for different active medical materials and substances in parallel can pose risks to patients’ and workers’ health. In this sense, causes of contamination and cross-contamination vary and can be the result of technical issues or any other deficiencies. Some of the main reasons for cross-contamination include but are not limited to:
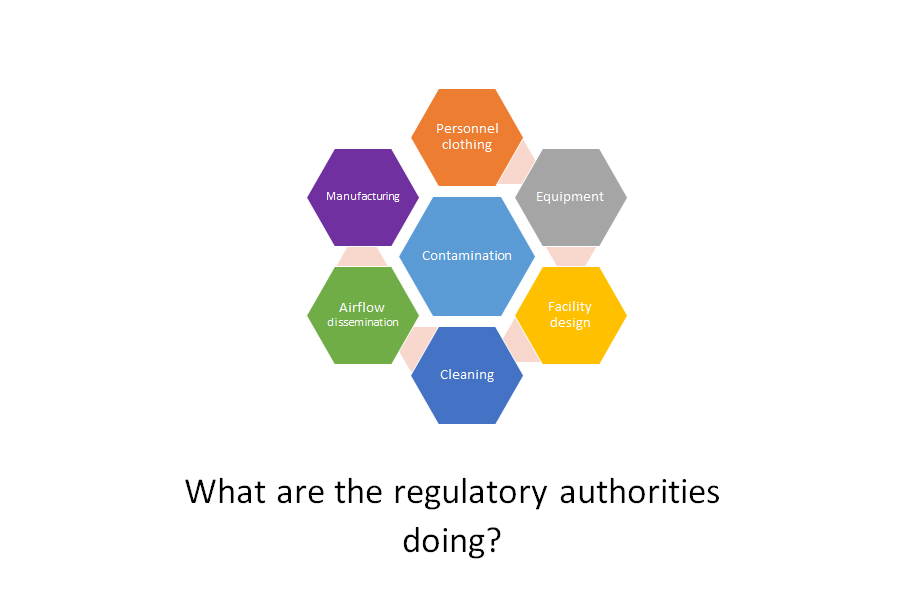
- possible antimicrobial carryover
- transition of active ingredients from one line to another which can easily happen through the uncontrolled dissemination of dust or gases
- transition of starting materials and genetic material or organisms from active substances
- carryover of extremely potent or small compounds in medicinal products
- residues of substances on equipment or operators’ clothing
The sources of contamination can be summarized in the following graphic:
In order to prevent cross-contamination from occurring and guarantee the wellbeing of workers and patients, regulators are scrutinizing manufacturing practices, tightening manufacturing requirements and initiating stricter GMP regulations. Nevertheless, some techniques and procedures may vary in different countries. And yet, regulators are still able to come up with globally-applicable recommendations and conditions which aim to enhance currently used methods. In this regard, EMA introduced changes to the regulations applied until 2015 and initiated improved “Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use.” Including new points and principles to several chapters and sections, the regulatory authorities request adoption of adequate design and operation of manufacturing facilities. Appropriate use of Quality Risk Management principles is also necessary in order to assess and control the cross-contamination risk that manufactured drugs may actually pose. What is more, there are different technical and organizational measures that need to be taken into account. This is important as it will ensure that all the premises and equipment are dedicated and specifically used for the purpose they are initially designed for.
Of course, the changes and requirements do not end here. And because they are so vital, we have also made changes to our existing GMP training, including the specifics of the new EMA guidelines. If you are interested in learning more about the subject matter and gain in-demand industry know-how, you can sign for our course here.
You can find a PDF version of this article here: https://crotraining.co.uk/wp-content/uploads/2016/10/Cross-contamination-in-Pharmaceutical-Manufacturing-and-Why-is-it-so-dangerous.pdf
References:
Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. Chapter 3 – Premise and Equipment; Chapter 5 – Production; Chapter 8 – Complaints and Product Recall http://ec.europa.eu/health/documents/eudralex/vol-4/index_en.htm [accessed: 20.10.2016]
FierceMarkets Custom Publishing “Cross-Contamination in Drug Manufacturing: The Regualtory Trends” http://www.ilcdover.com/sites/default/files/one_2_one_cross_contamination_final.pdf [accessed: 20.10.2016]