Although FDA regulations for the pharmaceutical industry have not altered that much for the last 30 years, expectations for a risk-based quality management are growing and shifting. The well-known set of ethical and scientific standards, provided by ICH and known as Good Clinical Practice, for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials resides in the foundation of every organization in this sector. But what exactly draws a link between GCP and Risk-Based Quality Management?
We have to look at the goals of GCP to answer that question. Its objectives are to provide public assurance that the rights, safety and welfare of participants in a certain clinical research or study are protected. Another purpose is to offer credible, informative and quality information. In order to do so, a risk based quality management system which is cost-efficient and time-saving should be applied to clinical trials. This way it can facilitate more consistent decision making when it comes to a certain project and can guarantee more adequate use of the available resources. Going further, a risk management is a systematic process which defines, estimates, controls, reviews, unveils and possibly mitigates risks associated with a clinical trial.
Nevertheless, the current practices conducted by sponsors and their agents (CROs etc.) lack substantial effectiveness. Hence, a more prioritized and systematic quality management is necessarily required in order to fully support the principles of GCP and to prevent problems such as: failure to identify priorities; poor risk identification and poor risk mitigation; lack of understanding of the impact of variability, flexibility and proportionality in the clinical trial process.
How can risk-based quality management help with ensuring GCP compliance?
Collecting the right information
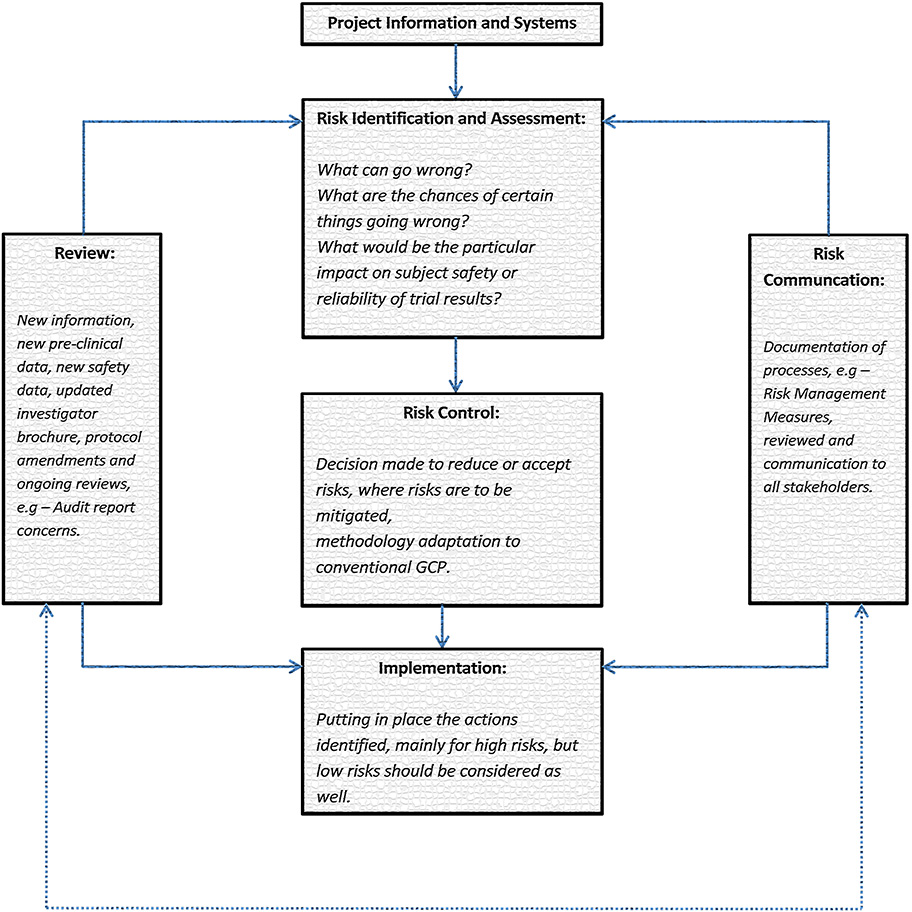
There are two levels to consider when collecting information that is needed for a proper risk identification and risk-based quality management. The first one is information obtained at system level. Precisely, this is the information associated with the environment and its systems. In other words a thorough information on the quality management system of the sponsor organization as well as the collaborators is collected. The second way is based on a project level where information regarding the investigational medicinal product(s), the project management and the demands of a clinical trial protocol to identify future risks is required.
Priorities
Still, before jumping to the phase when the risk is being identified and mitigated, it is first necessary to establish what really matters as well as to set the priorities that need to be addressed throughout a particular process. As a result, this will contribute to the identification of the risks that will be in the center of the risk based quality management process. After this, it is of a great significance to establish the acceptable variation or tolerance limits for the included clinical trial procedures because it would provide with a much better focus on the data measurement, collection and reporting. For instance, variation or tolerance limits could be applied to trial protocol procedures and GCP in order to extensively monitor the compliance/deviation from protocols.
Risk Review Cycle importance
Analyzing and summarizing the risk review cycle is pivotal. The delivered feedback is supposed to be focused on the measures of variability as well as their timing, assessment of deviations from tolerance limits or protocol requirements, and missing data all of which are associated with a clinical trial.
Mitigation/Acceptance of Risks
Finally, the risk-based quality management has the initial purpose to either reduce the risk to a level which is tolerable and reasonable or to accept it. So when risks are acceptable it means that they do not have too severe and dramatic threats to project objectives. On the other hand, when risks are not acceptable, they need to be reduced to another not so destructive level. How is this possible? By implementing efficient risk mitigation actions, for example:
Risk identification – Inadequate Facility, the facility does not have the required lab to run all the required parameters
Risk Mitigation/Acceptance – The sponsor needs to assess suitability of facilities against the protocol requirements and document during the site selection process and do so on an on-going basis. If the parameters required are essential and a priority for the study, the sponsor and the investigator should organise for the blood analyses to be done as per protocol by selecting a private laboratory.
In summary, clinical researched are about producing reliable information which will have an impact on a number of medical practices and products while simultaneously ensuring that safety, rights, and well-being of subjects are protected. The basic idea of risk-based quality management in terms of such studies is to identify possible risks on a continuous basis. Only this way 100% of effectiveness can be promised.
Pictures: https://higherlogicdownload.s3.amazonaws.com/RIMS/17c12695-b365-41d9-9fe6-ff93278e5e17/UploadedImages/Risk%20reduction.jpg



