The research environment is in a constant process of being affected by many external and internal factors. Things such as keeping privacy of patients, financial misbalances, not enough trained specialists, lack of trial participants, outdated trial methods, unreliable systems, incompliance with Good Clinical Practice (GCP) regulations, too much transparency, and the opposite, deprivation of data transparency are all very fragile corners in the Pharmaceutical, Medical and Clinical sector that can turn to problematical points quite easily. They are impactful enough...MORE >
- Share

- Share

Contemporary science is in a rush for quick results. However, when we talk about clinical researches and the Pharmaceutical industry, sometimes results do not come that fast. There is a number of different drawbacks and obstacles which, quite often, become an inseparable part of various research practices. Such holdbacks turn into the biggest challenges which every company is expected to deal with in the most adequate manner so that the study process can continue further on. Whether it is due...MORE >
- Share

Taking part in clinical trials happens voluntarily and it is entirely up to the subject to decide whether to continue their participation in the trial or to terminate it completely. Of course, such participations are surrounded by unavoidable formalities and requirements. One such requirement is for people to submit personal data to the board of researchers before the start date. This information along with the medical records of the patient are supposed to be adequately maintained and preserved during and...MORE >
- Share
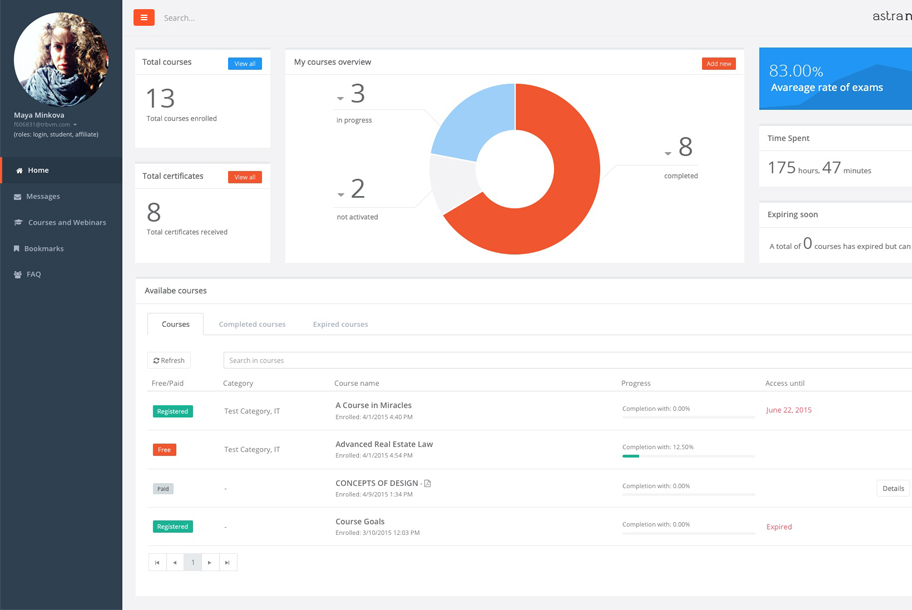
Astra Nova’s recent development is the launch of their new website and online learning platform - LMS (LEARNING MANAGEMENT SYSTEM). The new website presents awiderange of relevant opportunities across web technology, e-learning and continuing education in the health professions. Astra nova learning management system is designed to automate and streamline continuing medical education (CME) in medical associations, academic medical centres, education companies, and healthcare systems. Astra Nova is a UK-based training company dedicated to providing education solutions within the Pharmaceutical and Clinical Research...MORE >
- Share
Looking from a wider perspective, quality stands for a set of characteristics that some sort of service or a product is expected to have in order to be responsive to different requirements, laws, legislations and other formal documents. The concept of quality in the Pharmaceutical sector and clinical trials specifically, is a complex one. It can be defined in various manners depending on the used context and subject matter. Generally speaking, when it comes to clinical studies quality is explained...MORE >
- Share

In any industry which deals with product–development processes, quality assurance becomes an integral part of all of their procedures. When it comes to clinical trials and the Pharmaceutical, Medical and Clinical sector, making sure that high quality is achieved supports Good Clinical Practice (GCP) principles. What is more, it adds up to other quality practices, standards and requirements, thus having a direct impact on the overall relative industry performance. Ever since these particular branches have started to shape and contribute to...MORE >
- Share

Clinical trials transparency is among the hottest and most discussed topics which circulate around the Pharmaceutical, Medical and Clinical world. Even though it is considered to work in the best interest of clinical trial participants, the truth is in fact, quite different. This is so not because the easy accessible database reveals information which may jeopardize clients’ confidentiality, for example. It is rather because in some cases there is pivotal data about clinical trials results which is actually missing. What...MORE >
- Share

Good Storage Practices (GSPs) play an integral role in various Pharmaceutical and Pharmacovigilance-oriented companies, organizations and institutions. Each one of them is required to demonstrate not only efficient management but also efficient storage of pharmaceutical products. Such efficient storage of drugs is essential because it will that the potency and the physical integrity of medicaments are preserved and kept. What is more, GSPs are activities which generally prevent deterioration and ensure that the quality and safety of drugs are also...MORE >
- Share

Clinical trials transparency is understood to be part of all Good Clinical Practices (GCPs) and is further envisioned to work in the best interest of trial participants. But is it in the best interest of drug makers and medical researches as well or is the situation quite different? Looking in-depth, the European Medicines Agency (EMA) regulation regarding the disclosure of clinical trials information has the purpose to find the right balance between the public and research organizations. Undoubtedly, publishing studies–related...MORE >
- Share
Bringing to one’s attention the strict, Good Clinical Practice (GCP)- compliant and fast-pacing nature of clinical trials and the sponsors’ attempts to recruit as many suitable subjects as possible, in most of the cases the latter are seen to implement what traditionally works best for and in the Pharmaceutical industry. However, “what traditionally works best” may not be sufficient enough especially when there is a noticeable decrease in successful recruitment rates. What should be the next step then? Taking into...MORE >
