Our partners from Business Review Webinars have a message for you! This autumn we have lots of interesting webinars to keep you up to date with the latest research and developments in the pharmaceutical industry and you can watch them all from your very own home or office. We have industry experts sharing their latest products and research from leading companies such as Covance, Stevanato Group, Biovia, OSISoft and many more. You can have your say in polls, take part in surveys, and...MORE >
- Share
The Pharmaceutical and Clinical Research industries are part of a constantly evolving ecosystem that strives to improve and progress all the time. In an effort to pursue that endless strive for improvement, Astra Nova Training started working closely with BGO Software – a company with many years of expertise in the development of Healthcare software for a number of CROs from all around the world throughout the years. So far a number of such projects have been completed by BGO Software....MORE >
- Share

Keeping up to date with the latest research and information is vital for pharmaceutical professionals to ensure they are providing the best possible products and service. Webinars are a brilliant way to do this. As a knowledge sharing platform, they allow industry leaders to give insight and educate those working within the pharmaceutical sector. This keeps you up to date with areas such as drug discovery, drug development, drug formulation, clinical development, and clinical trials. Each of our webinar lasts approximately 60...MORE >
- Share

Clinical trials are at the heart of all advances in healthcare. The more effective treatments, drugs or combination of pharmaceuticals researchers discover, the bigger the chance of finding a cure becomes. In relation to this, FDA’s Center for Drug Evaluation and Research (CDER) plays a huge role in the medical field and its progress. Each year, CDER reviews and approves a number of novel drugs as well as therapeutic biological and other medical products which are safe and effective enough...MORE >
- Share

Subscriptions are becoming a growing trend in almost every industry. This comes as no surprise as behind this trend there is a number of benefits for both business and customers. Same applies to the Clinical and Research sector. As we know, in order to upgrade their knowledge and keep their skills sharp, practitioners look for the best online courses, seminars and consultancies that will help them accomplish all of this. With plenty of options out there, choosing the right training...MORE >
- Share

Ever since the first introduction of the Guide to Good Manufacturing Practice in 2001, there have been a number of updates occurring in the GMP setting. Because the manufacturing environment and the whole research landscape are continuously changing, revising the guidelines is simply inevitable. In many way, requirements of regulatory authorities tighten up, more advanced qualifications of personnel are asked for, stricter principles are discussed and implemented, and more computer systems as well as technology-driven procedures come into play. It...MORE >
- Share

The manufacturing environment and regulatory setting in Pharma are changing. There is a continuous adoption of new approaches when it comes to Good Manufacturing Practice (GMP) by researchers and other practitioners. Progressive technologies are coming into play as well, in attempt to streamline the landscape of drug manufacturing, the Clinical industry, the Research sector and healthcare in general. Consequently, clinging on to outdated requirements which do not apply to the current state of all of those fields becomes a practice...MORE >
- Share

The term Good Laboratory Practice or GLP was first introduced in 1970’s in Denmark and New Zealand. Soon after that, it was used by the Federal Drug Administration (FDA) and the Organisation for Economic Co-operation and Development (OECD), referring to a quality system of regulation and management practices or requirements in research laboratories. Good Laboratory Practice embodies different principles which are designed to ensure and promote consistency, quality, safety, reliability and integrity of chemicals during non-clinical and laboratory testing. Nevertheless,...MORE >
- Share

Poor quality pharmaceuticals and similar medical products pose great risks to patients and healthcare in general. On top of turning into a waste of time, effort and resources, in the end, they cannot deliver the intended treatment results and therapeutic effect. The main reason behind products’ inefficacy and hazardous nature is insufficient or uncontrolled manufacturing environment. In this regard, in order to prevent risks of infections, complications, degradation of medicine, cross-contamination or other negative impact before during and after production,...MORE >
- Share
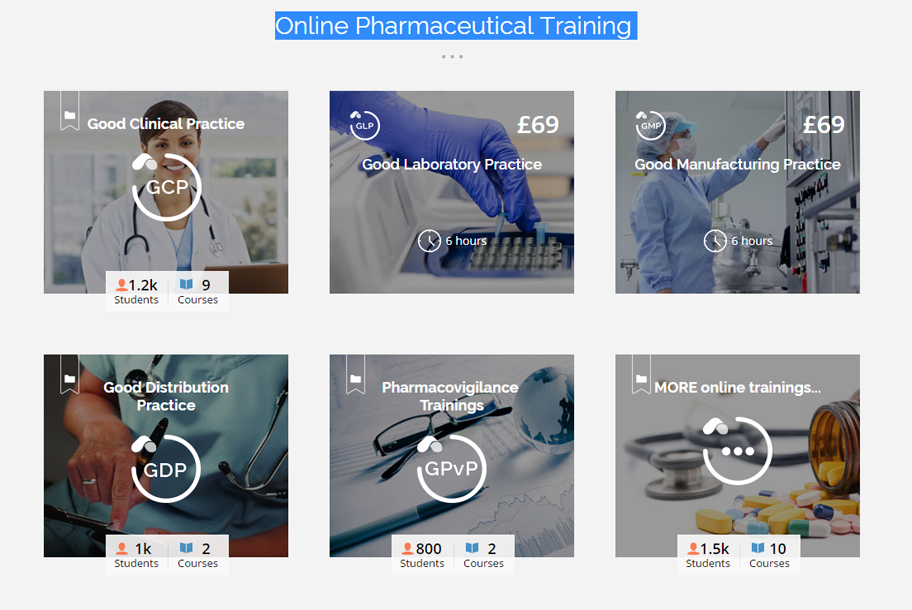
As an industry leading training and certification provider, Astra Nova provides bespoke online training programs, in-house training solutions, webinars and a number of free courses suitable for Clinical Research Organizations, Pharmaceutical companies and other institutions in the field of Biotechnology. With the new year just around the corner, we are now launching a Christmas campaign which delivers great offers and discounts on all of our individual and corporate certification courses. Gaining in-depth knowledge on ethics, regulatory requirements, guidelines, good practices, audits, informed consent, drug...MORE >

