GDP stands for many things, including Good Distribution Practice. Good Distribution Practice deals with the guidelines for the proper distribution of medicinal products for human use. GDP is defined as:
“The measures that need to be considered in the storage, transportation and distribution of any registered product /notified cosmetic and its related materials such that the nature and quality intended is preserved when it reaches the consumer”
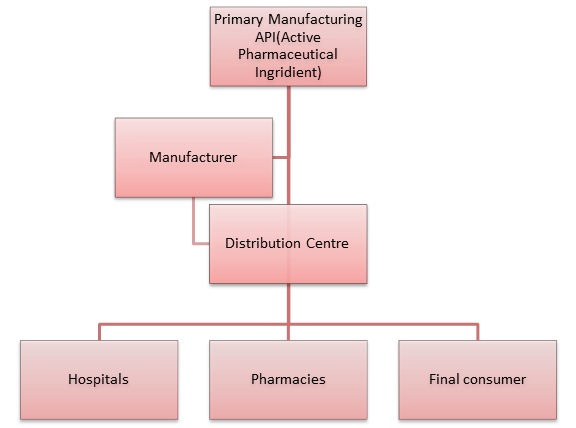
The GDP standards describe the distribution process from the manufacturing facilities to the end user, which makes it a part of the whole Quality Assurance process. On a global basis there are more than 30 different GDP implemented local regulations. Most of them are quite similar to the international requirements for storage, transportation etc., but in some countries like Brazil, Saudi Arabia, Argentina etc., because of specific cases and structures, there are also additional requirements that have to be followed.
Most important principles:
- Quality Management System(QMS): This is the system that describes every single step of the distribution process and according to GDP every distributor should have such in place.
- QMS should be adapted to all your internal and external processes.
- QMS should be fully documented
- A Responsible Person should be appointed
- The Change Control System-is a part of QMS
- A Management Review process should take place on a periodic basis and at least once a year
- Quality Risk Management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug product across the product lifecycle.
- A Corrective Action and Preventive Action (CAPA) process
Personnel and equipment:
There should be an adequate number of competent personnel involved in all stages of the wholesale distribution activities of medicinal products. The number of personnel required will depend on the volume and scope of activities. The organisational structure of the wholesale distributor should be set out in an organisation chart.
All equipment impacting on storage and distribution of medicinal products should be designed, located and maintained to a standard which suits its intended purpose. Planned maintenance should be in place for key equipment vital to the functionality of the operation.
Equipment used to control or to monitor the environment where the medicinal products are stored should be calibrated at defined intervals based on a risk and reliability assessment.
Training:
All personnel involved in the distribution process should be trained in GDP and depending on their role and responsibilities, job-specific trainings that ensure relevance and information on new practice development. A record of all qualification courses should be maintained and the effectiveness of trainings should be periodically assessed and documented. You can test a free demo of a full GDP training here: Good Distribution Practice
Need for implementation:
As the Pharmaceutical and Biotechnology industries world became global (derrived by the quest for ever-decreasing production costs), more API (Active Pharmaceutical Ingredients) and drug products are being manufactured in one region and are being transported/imported/exported to other regions around the globe. As a result, supply chain safety, validation and Good Storage and Distribution Practices became much more critical and relevant in this era. An uncontrolled or unsafe supply chain my lead to huge risks related to product safety and qualify that can easily lead to product recalls or even risk for patients health.